Our Research Active materials for heavy metal extraction from water
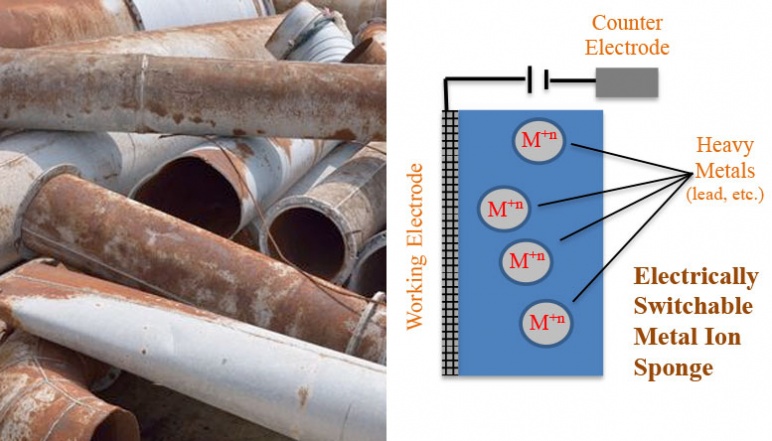
Image credit: iStock Images and Swager research team
Principal Investigator
Timothy Swager
- John D. MacArthur Professor of Chemistry
- Department of Chemistry
Timothy M. Swager is the John D. MacArthur Professor of Chemistry at the Massachusetts Institute of Technology. He is known for innovations in organic chemistry, polymers, and analytical science. A number of his contributions have focused on the development of materials and methods that allow for ultra-trace detection of chemicals and biological species. He has collaborated with Griffin in the development of dynamic nuclear polarization methods with the development of a number of hyperpolarization agents.
Challenge:
How can lead, mercury, and other heavy metals be extracted from contaminated water in a cost effective and localized way?
Research Strategy
- Engineered polymers to remove mercury and lead ions from water
- Designed a scalable water filter using these polymers
- Built energy efficiency and affordability into the design to allow access across income levels
Project description
The research team of this project created economically viable and scalable electroactive polymer compositions that have high affinities for soft heavy metal ions such as mercury and lead in their reduced state. The materials were active and could be switched by oxidation to a state wherein they had a greatly reduced affinity for the metal ions. The materials incorporated thioether groups that are established as being able to extract mercury from water. Different chemical structures were first evaluated at a small molecule level to determine if the redox active groups need to be directly incorporated into the metal binding group or can simply be proximate. The need for cyclic verses acyclic structures to bind the metal ions was also investigated. Cyclic structures have lower entropic energetic penalties with their organized binding of metal ions. Polymer composites were produced that incorporate structures that promote free volume and 3- dimensional ion diffusion in the polymer network.
These structures were formed by electrostatic assembly of the electroactive polymers in their oxidized cationic state with a novel polyelectrolyte that had been recently developed in the Swager group. The electrostatic assembly was also useful because reduction of the electroactive polymer resulted in an uncompensated charge from the sulfonate groups. This helped the material to actively recruit cationic metal ions and when combined with the high affinity of the incorporated thioethers, the system strongly bound mercury and lead. These materials can be studied as immobilized films or membranes on electrodes. These studies allowed the determination of how metal binding affects the electrochemical potential of the electroactive polymers. Additionally, by depositing films on interdigitated electrodes it was possible to see if the electrical conductivity is dependent on the target metal ions. The composite materials could also be synthesized as colloidal particles that can be cycled between their binding and release of metal ions by treatment with oxidants and reductants. The team also investigated if new materials that contain electron acceptors can be used to transiently charge the electroative materials under sunlight. The latter would open up many new opportunities as it could lead to systems that do not require access to electrical power.
Outcomes
- Created a polyaniline adsorbent that can selectively remove Hg(II) from water in its reduced state and release the contaminant again in its oxidized state and meets the US EPA standard of Hg(II) removal
- Established that the addition of sulfur chelating groups to polyaniline results in a higher uptake of contaminants in water
- Utilized the nanofiber geometry of polyaniline to attain faster kinetics
- Conducted molecular dynamics simulations to calculate the intermolecular binding energy between polyaniline and Hg2+ in order to better understand the adsorbent mechanism
Publications
Polyaniline nanofiber electrodes for reversible capture and release of mercury(II) from water
Kim, Yoonseob; Lin, Zhou; Jeon, Intak; Voorhis, Troy Van; Swager, Timothy M., Journal of the American Chemical Society, 2018
Dynamic self-correcting nucleophilic aromatic substitution
Wen Jie Ong and Timothy M. Swager, Nature Chemistry, 2018
Anion exchange membranes: Enhancement by addition of unfunctionalized triptycene poly(ether sulfone)s
Kim, Yoonseob; Moh, Lionel; Swager, Timothy, ACS Applied Materials & Interfaces, 2017
Janus emulsions for the detection of bacteria
Qifan Zhang, Suchol Savagatrup, Paulina Kaplonek, Peter H. Seeberger, and Timothy M. Swager, ACS Central Science, 2017
Additional Details
Impact Areas
- Water
Research Themes
- Water Purification & Desalination
- Technology & Commercialization
- Equity & Access
Year Funded
- 2016
Grant Type
- Seed Grant
Status
- Completed
