Our Research Using siderophore-conjugated microcins to combat foodborne pathogens
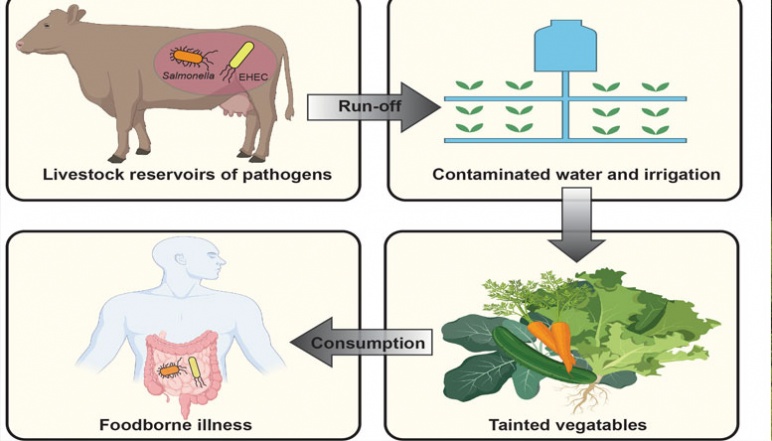
Pathogenic bacteria in cattle can contaminate agricultural products, leading to foodborne illnesses in humans. Credit: Nolan Research Team, created with BioRender.com

Cows grazing: livestock are a prevalent reservoir for pathogenic foodborne bacteria. Credit: Nolan Research Team
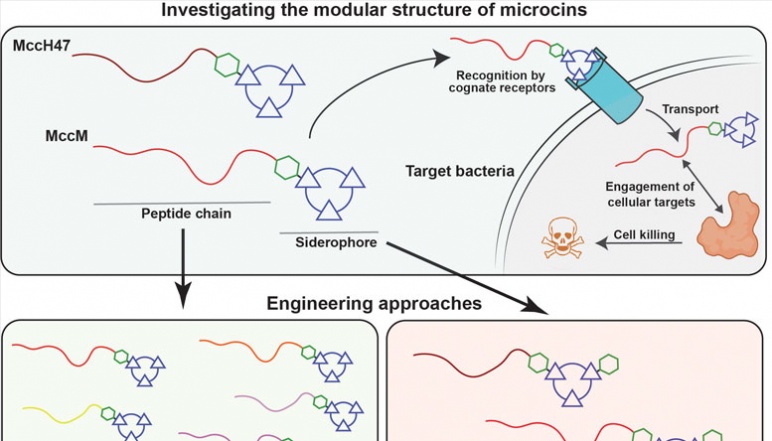
MccM and MccH47 have modular structures that allow for selectively entering certain bacteria and exerting antimicrobial activity. The Nolan lab will investigate the molecular basis for MccM/H47 biological activity and leverage the structural modularity to develop novel agents with improved potencies and selectivities. Credit: Nolan Research Team, created with BioRender.com
Principal Investigator
Elizabeth M. Nolan
- Ivan R. Cottrell Professor of Immunology
- Professor of Chemistry
- Department of Chemistry
Elizabeth M. Nolan is a Professor of Chemistry at MIT. Her current research interests address the chemistry and biology of human innate immunity and microbial pathogenesis. Her lab employs the toolkits of biological chemistry, inorganic chemistry, and microbiology to decipher the interplay between the human innate immune system and microbes, and to conceptualize and evaluate new strategies for preventing and treating microbial infections.
Challenge:
Can we control foodborne pathogens in livestock by using natural and engineered peptides with selective antimicrobial activity?
Research Strategy
- Investigate the structural characteristics of naturally occurring antimicrobial peptides that allow for selective targeting and biological activity
- Use engineered enzymes and peptide synthesis to manufacture peptides to specifically target pathogenic bacteria
- Discover novel peptide sequences which possess superior antimicrobial activity
Project description
Foodborne bacterial pathogens, such as Salmonella and enterohemorrhagic E. coli are a prevalent cause of human disease worldwide, imposing a severe economic burden and constituting a considerable health risk to young, elderly, or immunocompromised individuals. Livestock–particularly cattle–can contribute to the spread of these pathogens, which live in their guts. Thus, livestock are also a potential point of intervention for controlling the spread of foodborne pathogens. This seed grant will support our efforts to develop strategies to selectively target and kill pathogenic bacteria in the gut. We have identified two naturally occurring peptides produced by probiotic E. coli, Microcin M (MccM) and H47 (MccH47), that are important players in gut microbial competition as promising scaffolds for molecular engineering efforts. Each microcin is comprised of two functional components—a peptide providing antimicrobial activity and a siderophore, which targets the peptide to specific bacteria. Our group aims to understand the molecular basis for the selectivity and biological activity of MccM and MccH47. We will further use the understanding gained to develop novel microcin variants with improved antimicrobial potency and fine-tuned selectivity to target foodborne pathogens.
Outcomes
- Leveraged peptide synthesis to produce Microcin M in quantities large enough for functional evaluation
- Delved more deeply into how Microcin M enters cells through E. coli siderophore receptors—outer membrane proteins that recognize and transport small molecules called siderophores that are produced by E. coli and function in iron acquisition
- Gleaned new insights into the antibacterial role of the siderophores and how, once inside the cell, Microcin M kills E. coli
- Used bioinformatics to mine genomic databases and discovered approximately 200 new microcin peptides that can be further evaluated and developed to make novel antimicrobials with potential utility for the control of food-borne pathogens
Additional Details
Impact Areas
- Water
- Food
Research Themes
- Transforming Food Systems
Year Funded
- 2020
Grant Type
- Seed Grant
Status
- Completed
